How LEMTRADA Works
Proposed Mechanism of Action
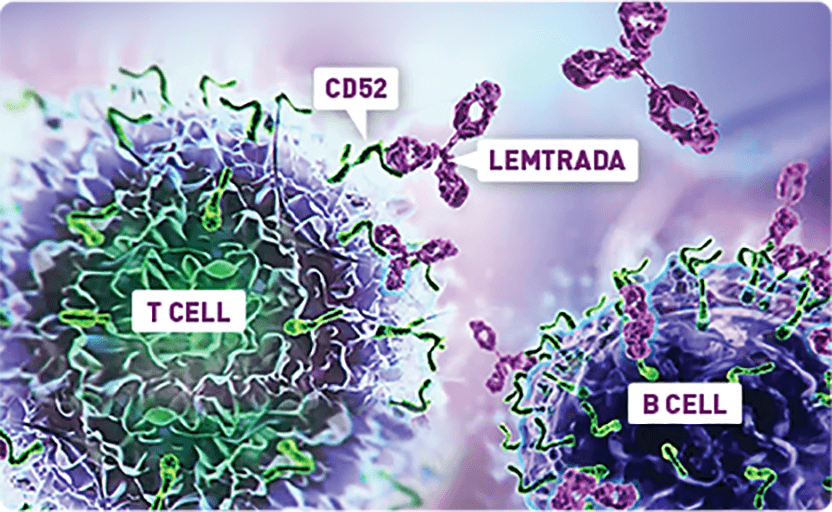
LEMTRADA depletes T and B cells, two key drivers thought to cause MS inflammation. The exact mechanism by which LEMTRADA exerts its therapeutic effects in MS is unknown.
LEMTRADA is a monoclonal antibody that selectively binds to CD52, highly expressed on lymphocytes (T and B cells), depleting these cells from circulation in the periphery. Other immune cells in the periphery have lower CD52 expression and are minimally affected.1,3,4
After Treatment With LEMTRADA
Repopulation of T and B cells occurs over time with innate immune cells remaining within normal ranges.1,4,5
- 5.0
- 4.5
- 4.0
- 2.0
- 1.5
- 1.0
- 0.5
- 0.0
- Months
- 0 Course 1
- 1
- 3
- 6
- 9
- 12 Course 2
- 15
- 18
- 21
- 24
Innate immune cells remained
within normal ranges4
-
After initial depletion of CD52-expressing
T and B cells, these cells slowly repopulate
over time. -
Innate immune cells (eosinophils,
neutrophils, basophils, and monocytes)
all remained in normal ranges.
- 1.0
- 0.8
- 0.6
- 0.4
- 0.2
- 0.0
- Months
- 0 Course 1
- 1
- 3
- 6
- 9
- 12 Course 2
- 13
- 15
- 18
- 21
- 24
~40%
of patients had total lymphocyte counts reach normal levels 6 months after each course.1
~80%
of patients reached normal
levels after 12 months.1
- B cells usually reach baseline levels by 6 months; T cells generally do not reach baseline levels by 12 months.1
- The proportion of regulatory T cells was elevated following LEMTRADA treatment, gradually returned toward baseline levels, but remained elevated at month 12.4
Serum concentrations of LEMTRADA were generally undetectable within approximately 30 days following each treatment course.1
Choosing LEMTRADA
Healthcare professionals share perspectives on LEMTRADA and what makes it an appropriate choice for some.
DR. RANY ABURASHED: So when I think about the ideal LEMTRADA patient, typically there’s a patient who we’ve had on at least two drugs and whose disease has shown that it’s being stubborn and it wants to keep fighting.
DR. HEIDI CRAYTON: It can be people who are having relapses despite being on whatever therapy they’re on.
DR. RANY ABURASHED: And it’s typically a patient whose physical attacks are pretty clear. And that’s kind of the target that I choose for my LEMTRADA cases.
DR. RANY ABURASHED: So the idea with LEMTRADA and what we’re trying to do with LEMTRADA, is we’re trying to take a group of cells that we think are attacking the brain and we’re trying to essentially destroy them. And then we’re going to allow your body to regrow those cells under the hope that they will not attack the brain again. What you see is, approximately six months after we give this drug, you start to see the B cells start to come back at a slow rate and then approximately a year after, you start to see T cells start to come back. We monitor this very carefully because it gives us a little bit of an idea of what risk they may be at for infection.
DR. HEIDI CRAYTON: So, the dosing of LEMTRADA sometimes a little difficult for people to kind of wrap their head around. People are used to doing regimens daily, several times a week, monthly.
DR. RANY ABURASHED: You take this drug eight days over a two-year period. So, patients will come in in year one, we give them five days of an infusion. So, Monday through Friday, they will be infused. The following year, exactly one year later, we bring them back in again. We do three days of the, of the LEMTRADA infusion.
CARRIE BLIZZARD: LEMTRADA can cause serious side effects during infusion or up to 24 hours longer after you received LEMTRADA. Tell your healthcare provider immediately if you experience any discomfort during or after your infusion. You may also be given other medications before or after the infusion to try and reduce your chances of getting these reactions or to treat them after they happen.
DR. RANY ABURASHED: After a patient is infused with LEMTRADA, one of the most important things we do is to monitor patients’ blood and urine every month.
CARRIE BLIZZARD: You will need monthly blood and urine tests, self-checks, and yearly skin exams. These help to monitor possible side effects that can show up months or even years after your last infusion, including autoimmune side effects and some kinds of cancers, including skin cancer melanoma. It is important to have your blood and urine tested even if you’re feeling well and do not have any symptoms from LEMTRADA or your MS. This may help your healthcare provider find side effects early.
LYNN GOBEILLE: With the lab monitoring, um it’s important that patients continue to, um, have that done, regardless of how well you’re feeling. Your monitoring is going to continue for at least forty-eight months after the last infusion. So, it could be forty-eight months, it could be longer.
DR. RANY ABURASHED: It’s very important for us to monitor them monthly. In doing so, we can help catch any potential side effect very early and, if it happens, we can then treat it.
DR. RANY ABURASHED: Some patients are very very conservative in terms of their goals, and they say, “I don’t want anything that has risk.” And at that point it’s very important to give them the data on what these drugs do and give them the data on what we know. We have a lot of information of this, on these drugs. So, it’s imperative that I work with them, and I come up with the best treatment plan for them.
DR. HEIDI CRAYTON: It’s very important to explain risks and benefits. Um, I like to talk about what the medicine is proposed to do and why I want to implement that particular medication. We really talk about the fact that these are the things you’re going to read about, these are the things that are listed. So, once they get through that part then we, you know, we talk about monitoring. We’re educating them on what to look for and we’re monitoring blood and urine every month for forty-eight months after the last infusion. There are several options to do that. People can either go to their healthcare provider’s office, they can go to a freestanding LabCorp Quest. And people have to really buy into the monitoring with LEMTRADA. They have to be willing to be a partner, an ongoing partner in this relationship. Um, oftentimes people down the road will feel really good and they may not feel that they have to stay connected to the healthcare team. But I explain that some of the things we are monitoring for in terms of other autoimmunity can happen down the road, years down the road.
DR. RANY ABURASHED: I tell patients all the time that we are climbing a mountain and I’m kind of in front and I’m the guide. And it’s us moving together as a team as we try to get to the top of this mountain. Though there’s a lot that we don’t understand, there’s a ton we do.
DR. HEIDI CRAYTON: It takes a village. It definitely takes a village.
LYNN GOBEILLE: You know, partner with your healthcare provider. They are a part of your team and you’re the boss.
INDICATION
LEMTRADA is a prescription medicine used to treat relapsing forms of multiple sclerosis (MS), to include relapsing-remitting disease and active secondary progressive disease, in adults. Since treatment with LEMTRADA can increase your risk of getting certain conditions and diseases, LEMTRADA is generally prescribed for people who have tried 2 or more MS medicines that have not worked well enough. LEMTRADA is not recommended for use in patients with clinically isolated syndrome (CIS). It is not known if LEMTRADA is safe and effective for use in children under 17 years of age.
IMPORTANT SAFETY INFORMATION
LEMTRADA can cause serious side effects including:
Serious autoimmune problems: Some people receiving LEMTRADA develop a condition where the immune cells in your body attack other cells or organs in the body (autoimmunity), which can be serious and may cause death. Serious autoimmune problems may include:
- Immune thrombocytopenic purpura (ITP), a condition of reduced platelet counts in your blood that can cause severe bleeding that may cause life-threatening problems. Call your healthcare provider (HCP) right away if you have any of the following symptoms: easy bruising, bleeding from a cut that is hard to stop, coughing up blood, heavier menstrual periods than normal, bleeding from your gums or nose that is new or takes longer than usual to stop, small, scattered spots on your skin that are red, pink, or purple.
- Kidney problems called anti-glomerular basement membrane disease, which, if not treated, can lead to severe kidney damage, kidney failure that needs dialysis, a kidney transplant, or death. Call your HCP right away if you have any of the following symptoms: swelling of your legs or feet, blood in the urine (red or tea-colored urine), decrease in urine, fatigue, coughing up blood.
It is important for you to have blood and urine tests before you receive, while you are receiving and every month for 4 years or longer, after you receive your last LEMTRADA infusion.
Serious infusion reactions: LEMTRADA can cause serious infusion reactions that may cause death. Serious infusion reactions may happen while you receive, or up to 24 hours or longer after you receive LEMTRADA.
- You will receive your infusion at a healthcare facility with equipment and staff trained to manage infusion reactions, including serious allergic reactions, and urgent heart or breathing problems. You will be watched while you receive, and for 2 hours or longer after you receive, LEMTRADA. If a serious infusion reaction happens while you are receiving LEMTRADA, your infusion may be stopped.
Tell your HCP right away if you have any of the following symptoms of a serious infusion reaction during the infusion, and after you have left the healthcare facility:
- swelling in your mouth or throat
- trouble breathing
- weakness
- fast, slow, or irregular heartbeat
- chest pain
- rash
To lower your chances of getting a serious infusion reaction, your HCP will give you a medicine called corticosteroids before your first 3 infusions of a treatment course. You may also be given other medicines before or after the infusion to try to reduce your chances of having these reactions or to treat them if they happen.
Stroke and tears in your arteries that supply blood to your brain (carotid and vertebral arteries): Some people have had serious and sometimes deadly strokes and tears in their carotid or vertebral arteries within 3 days of receiving LEMTRADA. Get help right away if you have any of the following symptoms that may be signs of a stroke or tears in your carotid or vertebral arteries: drooping of parts of your face, weakness on one side, sudden severe headache, difficulty with speech, neck pain.
Certain cancers: Receiving LEMTRADA may increase your chance of getting some kinds of cancers, including thyroid cancer, skin cancer (melanoma), and blood cancers called lymphoproliferative disorders and lymphoma. Call your HCP if you have the following symptoms that may be a sign of thyroid cancer: new lump, swelling in your neck, pain in front of neck, trouble swallowing or breathing, hoarseness or other voice changes that do not go away, cough that is not caused by a cold.
Have your skin checked before you start receiving LEMTRADA and each year while you are receiving treatment to monitor for symptoms of skin cancer.
Because of risks of autoimmunity, infusion reactions, and some kinds of cancers, LEMTRADA is only available through a restricted program called the LEMTRADA Risk Evaluation and Mitigation Strategy (REMS) Program.
Do not receive LEMTRADA if you:
- are allergic to alemtuzumab or to any of the inactive ingredients in LEMTRADA
- are infected with human immunodeficiency virus (HIV)
- have an active infection
Thyroid problems: Some patients taking LEMTRADA may get an overactive thyroid (hyperthyroidism) or an underactive thyroid (hypothyroidism). Call your HCP if you have: excessive sweating, unexplained weight loss, unexplained weight gain, fast heartbeat, eye swelling, nervousness, feeling cold, worsening tiredness, constipation.
Low blood counts (cytopenias): LEMTRADA may cause a decrease in some types of blood cells. Some people with these low blood counts have increased infections. Call your doctor right away if you have symptoms of cytopenias such as: weakness, chest pain, yellowing of the skin or whites of the eyes (jaundice), dark urine, fast heartbeat.
Inflammation of the liver: Call your HCP right away if you have symptoms such as unexplained nausea, stomach pain, tiredness, loss of appetite, yellowing of skin or whites of eyes, or bleeding or bruising more easily than normal.
Hemophagocytic lymphohistiocytosis: LEMTRADA may increase the risk of overactivity of the immune system that can be fatal if not diagnosed and treated early. If you experience symptoms such as fever, swollen glands, or skin rash, contact your HCP right away.
Thrombotic thrombocytopenic purpura (TTP): LEMTRADA may cause blood clotting problems that can be fatal. Call your HCP right away if you experience symptoms such as: purplish spots on skin or in mouth due to bleeding under skin, yellowing of skin or whites of eyes (jaundice), feel tired or weak, very pale skin, fever, fast heart rate or short of breath, headache, speech changes, confusion, vision changes, seizure, low amount of urine or dark or bloody urine, stomach pain, nausea, vomiting, or diarrhea.
Autoimmune encephalitis (AIE): LEMTRADA may cause AIE, a brain disorder which may include symptoms that seem like an MS relapse. Call your HCP right away if you have any of the following symptoms: personality changes, mood changes, seeing things that are not there (hallucinations), agitation, short term memory loss, confusion, movement disorders, or seizures.
Bleeding disorder (acquired hemophilia A): LEMTRADA may cause acquired hemophilia A. Call your HCP right away if you have any of the following symptoms: bruising, nose bleeds, bleeding from a cut that may take longer than usual to stop, painful or swollen joints, blood in urine, dark or bloody stools.
Serious infections: LEMTRADA may cause you to have a serious infection while you receive and after receiving a course of treatment. Serious infections may include:
- listeria. People who receive LEMTRADA have an increased chance of getting a bacterial infection called listeria, which can lead to significant complications or death. Avoid foods that may be a source of listeria or make sure foods are heated well.
- herpes viral infections. Some people taking LEMTRADA have an increased chance of getting herpes viral infections. Take medicines as prescribed by your HCP to reduce your chances of getting these infections.
- tuberculosis. Your HCP should check you for tuberculosis before you receive LEMTRADA.
- hepatitis. People who are at high risk of, or are carriers of, hepatitis B (HBV) or hepatitis C (HCV) may be at risk of irreversible liver damage.
These are not all the possible infections that could happen while on LEMTRADA. Call your HCP right away if you have symptoms of a serious infection such as fever or swollen glands. Talk to your HCP before you get vaccinations after receiving LEMTRADA. Certain vaccinations may increase your chances of getting infections.
Progressive multifocal leukoencephalopathy (PML): A rare brain infection that usually leads to death or severe disability has been reported with LEMTRADA. Symptoms of PML get worse over days to weeks. It is important that you call your doctor right away if you have any new or worsening medical problems that have lasted several days, including problems with thinking, eyesight, strength, balance, weakness on one side of your body, using your arms or legs.
Inflammation of the gallbladder without gallstones (acalculous cholecystitis): LEMTRADA may increase your chance of getting inflammation of the gallbladder without gallstones, a serious medical condition that can be life-threatening. Call your HCP right away if you have stomach pain or discomfort, fever, nausea, or are vomiting.
Swelling of lung tissue (pneumonitis): Some people have had swelling of the lung tissue while receiving LEMTRADA. Call your HCP right away if you have shortness of breath, cough, wheezing, chest pain or tightness, or are coughing up blood.
Before receiving LEMTRADA, tell your HCP if you:
- have bleeding, thyroid, or kidney problems
- have a recent history of infection
- are taking a medicine called Campath® (alemtuzumab)
- have received a live vaccine in the past 6 weeks before receiving LEMTRADA or plan to receive any live vaccines. Ask your HCP if you are not sure if your vaccine is a live vaccine.
- are pregnant or plan to become pregnant. LEMTRADA may harm your unborn baby.You should use birth control while receiving LEMTRADA and for 4 months after your course of treatment.
- are breastfeeding or plan to breastfeed. You and your HCP should decide if you should receive LEMTRADA or breastfeed.
Tell your HCP about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. LEMTRADA and other medicines may affect each other, causing side effects. Especially tell your HCP if you take medicines that increase your chance of getting infections, including medicines used to treat cancer or to control your immune system.
The most common side effects of LEMTRADA include:
- rash
- headache
- thyroid problems
- fever
- swelling of your nose and throat
- nausea
- urinary tract infection
- feeling tired
- trouble sleeping
- upper respiratory infection
- herpes viral infection
- hives
- itching
- fungal infection
- joint pain
- pain in your arms or legs
- back pain
- diarrhea
- sinus infection
- mouth pain or sore throat
- tingling sensation
- dizziness
- stomach pain
- sudden redness in face, neck, or chest
- vomiting
Tell your HCP if you have any side effect that bothers you or that does not go away. These are not all the possible side effects of LEMTRADA.
Please see accompanying full Prescribing Information/Medication Guide, including serious side effects.
Clinical Study Patient Characteristics
At entry, the average CARE-MS II patient who previously experienced disease activity while on a prior therapy is represented by the characteristics below1,2:
CARE-MS II Baseline Characteristics
|
||||||||||||||||||||||||||||||||||||||||||
CARE-MS II:
A 2-year, randomized, open-label, rater-blinded, phase III study that included RMS patients (N=628) with EDSS scores of ≤5.0, who had experienced ≥2 relapses in the previous 2 years, with at least 1 relapse in the prior year and at least 1 relapse while on interferon beta or glatiramer acetate therapy after at least 6 months of treatment. Patients received LEMTRADA (12 mg intravenous infusion once daily for 5 days at month 0 and then once daily for 3 days at month 12; n=426) or Rebif (subcutaneous interferon beta-1a 44 μg, 3 times per week for 2 years; n=202).1,2
CARE-MS: Comparison of Alemtuzumab and Rebif Efficacy in Multiple Sclerosis; EDSS: Expanded Disability Status Scale; Gd+: gadolinium-enhancing.
